Help Your Discovery of COVID-19 mRNA Vaccine with TransGen
COVID-19 pandemic has enabled mRNA vaccines to race to the front of pack due to its faster speed and easier scalability of manufacture than conventional vaccines. mRNA vaccines topped the MIT Technology Review’s 2021 list of 10 Breakthrough Technologies because of their great potential to be new therapeutics against not only infectious diseases like SARS-CoV-2 virus but also non-infectious diseases, such as cancer and autoimmune disease. For those who are curious about the frontier research and development or already scientists in this field, here is a brief introduction and summary of the history, science, production, advantages, and challenges in the future of mRNA vaccines, hoping that TransGen will facilitate mRNA vaccine development to fight against COVID-19 with our strengths.

1Decades of Research Efforts Before COVID-19
Researchers have been persistently making efforts to develop mRNA vaccines for decades. The study of mRNA traced back to 1961 when it was first isolated. More than 20 years later, the idea of mRNA-based drug was raised when it was successfully transfected and expressed in eukaryotic cells. However, concerns related to mRNA instability, higher innate immunogenicity, and inefficient in vivo drug delivery became a brake to the development in mRNA-based vaccines. Nevertheless, studies on structural and functional characteristics of eukaryotic mRNA and its metabolism in the initial decades paved the way for the lasted discoveries and improvements of mRNA vaccines. In addition, mRNA delivery is also the groundwork for today’s mRNA vaccines, which was initiated in the 1970s by Robert Langer, the David H. Koch Institute Professor at MIT, a member of the Koch Institute, and one of the founders of Moderna. The breakthrough in mRNA vaccines was achieved in 2005, made by Dr Drew Weissman and Kariko (a senior vice president at BioNTechn now) at the University of Pennsylvania (Penn). They discovered that the use of modified nucleosides increase the stability of the mRNA and production of antigen, while decrease the innate immunogenicity. Since then, interests in mRNA vaccines exploded and mRNA platform came to a new era. Currently, two mRNA vaccines obtained the regulatory authorization or approval for use in humans, and they are the Pfizer/BioNTech and Moderna COVID-19 vaccines.
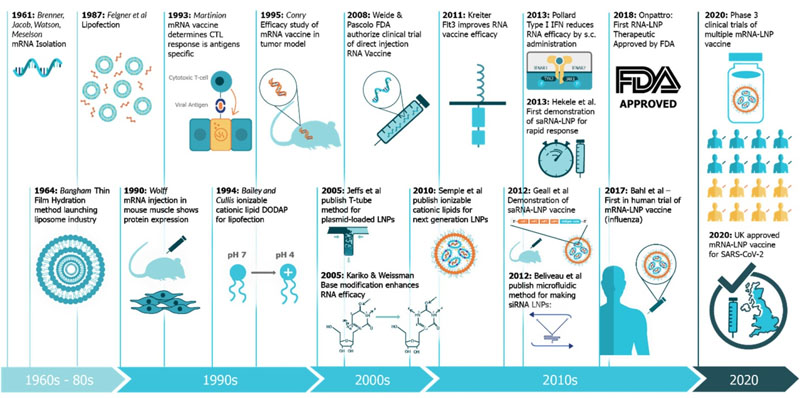
Figure 1. A timeline of innovations that have contributed to mRNA vaccines and associated technologies
2How COVID-19 mRNA Vaccines Work
Briefly, mRNA vaccines deliver the open-reading frame (ORF) of immunogen to host cell cytoplasm and make it expressed in host cell machinery, triggering humoral and cellular immune responses to provide protection against infectious pathogens or cancer.
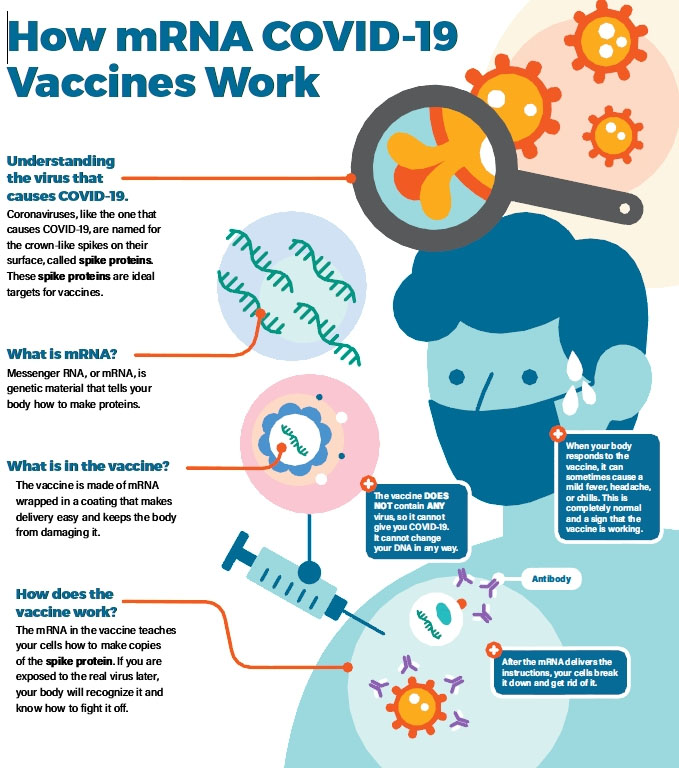
Figure 2. How COVID-19 mRNA Vaccines Work
3How mRNA Vaccines Are Produced
Related Products
The development and production of mRNA vaccines is simpler than traditional vaccines and DNA vaccines, and it is more standardized in produce target antigens and select/ produce vaccine antigens.
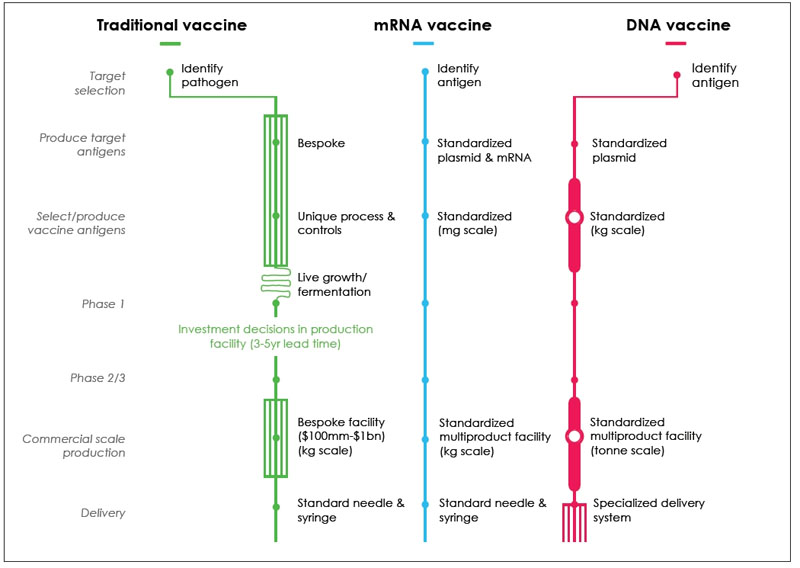
Figure 3. Comparison of Production Processes for Traditional, mRNA and DNA vaccines
Typically, the development and production process of mRNA vaccines is as follows [Figure 4]:
1. Screening and isolating target pathogens
2. Obtaining the genome of the pathogen and antigen(s) by sequencing (such as NGS technology), bioinformatics, and computational approach
3. Obtaining candidate vaccine antigen sequences
4. In silico design of mRNA vaccines
5. Generation of plasmid DNA (pDNA) template through molecular cloning or synthesis.
6. In vitro transcription from linearized pDNA plasmid by RNA polymerase
7. Modification and purification of mRNA
8. Formulation of mRNA vaccines with the delivery system.
9. Validation of mRNA vaccines by in-process analytic and potency tests
10. Large-scale production of the final mRNA vaccines
11.Delivery of mRNA vaccines for vaccination
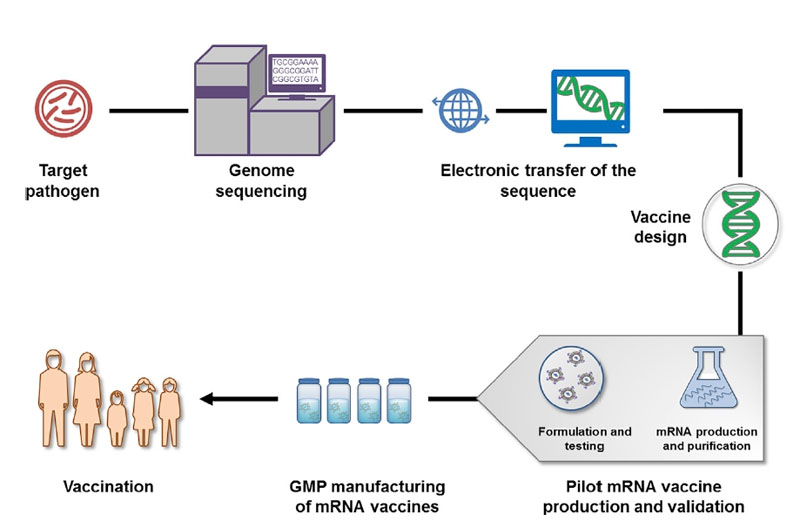
Figure 4. Schematic Illustration of mRNA Vaccine Production
4Advantages Over Other Vaccines
mRNA vaccines have advantages on safety, efficacy, and production over DNA vaccines and traditional vaccines (which include subunit, live attenuated and inactivated vaccines). mRNA does not cause insertional mutagenesis in the genome of the host cells or produce infectious particles; also, its controllable half-life in vivo, modulable innate immunogenicity, and the degradation under normal cellular conditions improve the safety profile. mRNA can be more stable and translatable through various modifications; when being formulated into carrier molecules, it can have great in vivo delivery efficiency, increasing its uptake and expression in the cytosol; besides, as the minimal hereditary material, mRNA does not cause anti-vector immunity, making it possible for the mRNA vaccines being administered repeatedly. The production of mRNA vaccines can be fast, low-cost, and scalable because the in vitro transcription reactions of mRNA could have high yields.
5Directions Researchers Are Still Working on
Related Products
However, there are also some challenges and concerns in the widespread therapeutic use of mRNA vaccines.
1) SAFETY
Though mRNA vaccines have been considered a relatively safe vaccine format because of the above stated advantages, side effects are non-negligible, which range from mild or modest pain, redness, swelling at the injection site and the tiredness, headache, muscle pain, chills, fever, nausea after injection within a few days to more serious or long-lasting side effects, such as anaphylaxis. Researches are working on the optimal balance between immune and inflammatory activation by optimizing constructs, formulations, delivery systems, clinical trials design, and manufacturing processes.
2) STABILITY AT HIGHER TEMPERATURES
The stringent storage and transportation conditions at low temperature (around -70℃ and -20℃) could slow the distribution and massive immunization of the mRNA vaccines against COVID-19.
The temperature requirements for storage and transportation for Pfizer/BioNTech’s vaccine are the most stringent: -80℃ to -60℃ for up to 6 months;2℃ to 8℃ for up to five days ; -25°C to -15°C for up to 2 weeks before mixing with a saline diluent.
Moderna COVID‑19 Vaccine can be stored frozen between -25º to -15ºC; between 2° to 8°C for up to 30 days prior to first use. Unpunctured vials may be stored between 8° to 25°C for up to 12 hours.
Although, the German company Curevac launched the vaccines later than Pfizer/BioNTech and Moderna, the vaccines demonstrate higher stability at 5°C for 3 months and up to 24 hours as ready-to-use vaccine when stored at room temperature.
Continual efforts are made to increase the stability at higher temperatures and shelf-life. Freeze-drying technique and optimized formulation enabled the vaccines to remain active at 5-25°C for 3 years and 60°C for 6 months. Recent stability study on liquid form of mRNA vaccines against COVID-19 in mice can maintain delivery efficiency at 4°C and 25C for at least one week. The advances in this area will solve the realistic problem faced by rural areas and developing countries.
3) DURATION OF PROTECTION AGAINST COVID-19
Current mRNA vaccines are applied in a two-dose immunization regimen with a dose of 12μg to 100μg in each dose. This administration format results from the waning immune response after vaccine injection which has aroused widespread concern over a possible short duration of vaccine protection. A single injection with lower amount of dose is more practical for long-term immunization strategy.
Emerging viral variants are another concern of vaccine efficacy. Considering the vaccine inefficacy interrelated with mutations in the target proteins, it is suggested to carefully select and design immunogen for the mRNA-based vaccine development.
6How TransGen Facilitates the Development
Currently, with the emergency need for COVID-19 vaccines worldwide, many companies and institutions all over the world have invested heavily to establish mRNA vaccine platforms and improve the current technologies. As a qualified supplier of biological reagents, TransGen provides a series of related products to assist the research, development, and manufacture of mRNA vaccines.
Reference
[1] MIT Technology Review, 2021. https://www.technologyreview.com/2021/02/24/1014369/10-breakthrough-technologies-2021/#messenger-rna-vaccines
[2] N Pardi, M Hogan, F Porter, et al. mRNA vaccines — a new era in vaccinology. Nat Rev Drug Discov. 2018 Apr;17(4):261-279. doi: 10.1038/nrd.2017.243.
[3] C Krienke, L Kolb, et al. A noninflammatory mRNA vaccine for treatment of experimental autoimmune encephalomyelitis. Science 2021, 371, 145–153
[4] A K Blakney, S Ip, et al. An Update on Self-Amplifying mRNA Vaccine Development. Vaccines 2021, 9, 244. https://doi.org/10.3390/vaccines9030244
[5] M M Rahman, N Zhou, et al. An Overview on the Development of mRNA-Based Vaccines and Their Formulation Strategies for Improved Antigen Expression In Vivo. Vaccines 2021, 9, 244. https://doi.org/10.3390/vaccines9030244
[6] S Xu ,K Yang, et al. mRNA Vaccine Era—Mechanisms, Drug Platform and Clinical Prospection. Int. J. Mol. Sci. 2020, 21, 6582; doi:10.3390/ijms21186582
[7] https://news.mit.edu/2020/rna-vaccines-explained-covid-19-1211
[8] https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/mrna.html
[9] G Maruggi, C Zhang, J Li, J B Ulmer, et al. mRNA as a Transformative Technology for Vaccine Development to Control Infectious Diseases. Mol Ther. 2019 Apr 10;27(4):757-772. doi: 10.1016/j.ymthe.2019.01.020.
[10] Coronavirus disease (COVID-19): Vaccines. https://www.who.int/news-room/q-a-detail/coronavirus-disease-(covid-19)-vaccines
[11] E Rachlin and M Watson M, 2017. mRNA Vaccines: Disruptive Innovation in Vaccination, Moderna, Corporate Headquarters
[12]https://www.gavi.org/vaccineswork/4-things-about-mrna-covid-vaccines-researchers-still-want-find-out
[13]https://www.cdc.gov/vaccines/covid-19/info-by-product
[14]https://www.sciencemag.org/news/2020/11/temperature-concerns-could-slow-rollout-new-coronavirus-vaccines
[15] N N Zhang, X F Li, et al. A Thermostable mRNA Vaccine against COVID-19. Cell 2020 182, 1271–1283. https://doi.org/10.1016/j.cell.2020.07.024
[16] Q Hang, K Ji, et al. A single-dose mRNA vaccine provides a long-term protection for hACE2 transgenic mice from SARSCoV-2. NATURE COMMUNICATIONS 2021, 12:776. https://doi.org/10.1038/s41467-021-21037-2











